

Biopharmaceutical & Biotech
Empowering with premium products
High-quality ingredients are increasingly vital in the biopharma and biotech markets, transforming the pharmaceutical industry and benefiting patients.
These ingredients are essential for developing innovative therapies and ensuring higher efficacy and safety standards.
As demand for personalized medicine and biologics grows, reliable and cutting-edge ingredients become critical, accelerating drug development and enhancing patient outcomes.

If you have any questions about our products, please contact:

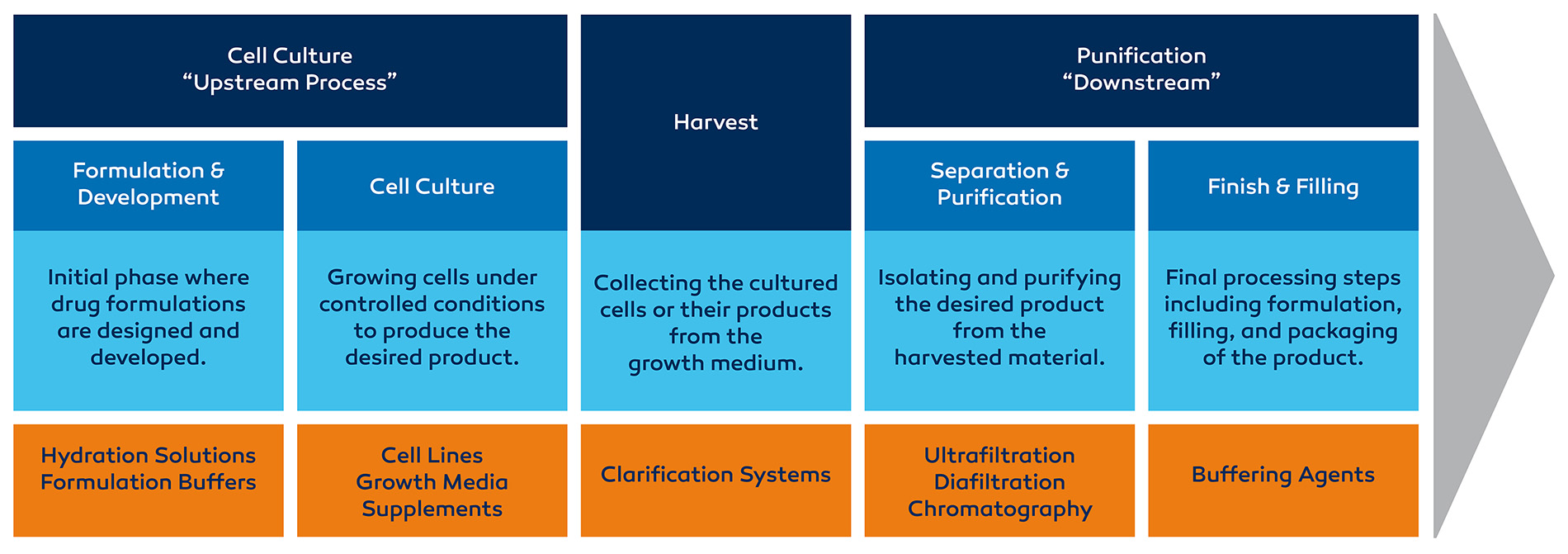
Cell Culture - “Upstream Process”
Formulation & Development
Initial phase where drug formulations are designed and developed.
Hydration Solutions
Formulation Buffers
Cell Future
Growing cells under controlled conditions to produce the desired product.
Cell Lines
Growth Media
Supplements
Harvest
Collecting the cultured cells or their products from the growth medium.
Clarification Systems
Purification - “Downstream”
Separation & Purification
Isolating and purifying the desired product from the harvested material.
Ultrafiltration
Diafiltration
Chromatography
Finish & Filling
Final processing steps including formulation, filling, and packaging of the product.
Buffering Agents
We are proud to collaborate with best-in-class ingredient suppliers to achieve our goals in the biopharma and biotech markets. Our partnerships ensure we deliver innovative and high-quality solutions, driving advancements in the pharmaceutical industry and improving patient outcomes.
About Biospectra
BioSpectra is a manufacturer of cGMP Fine Chemicals, located in Stroudsburg, Bangor and Wind Gap, PA, USA. BioSpectra manufactures a unique line of cGMP Fine Chemicals for use in pharmaceutical processes and finished drug products used by global BioPharma, Bio Contract Manufacturers a.k.a. Bio-CMOs (for APIs and Finished Drug Products), and Biochemical Companies. This includes bulk Biological cGMP Buffers, large volume cGMP Bio-Buffer Solutions, cGMP Process Fine Chemicals, cGMP Chlorinated Amino Acids, highly purified Parenteral Grade Carbohydrates and Carbohydrate (Dextran) polymers, as well as other novel and compendial Excipients.


Fine Chemicals
Navigating Global Supply Chains for Intermediates, Process Chemicals, and Active Pharma Ingredients (API)

About Nordmann



